Porphyrin chemistry and spectroscopy
1. Aromatically π-extended porphyrins
Synthetic chemistry and spectroscopy of porphyrins is an important research direction in our laboratory. In collaboration with Anderi Cheprakov (Moscow State University, Russia) we developed a general synthetic approach to porphyrins with the aromatically extended π-system. This is a unique class of porphyrinoids with photophysical properties that are valuable for imaging applications. Tetrabenzo- [1], tetranaphtho-[2, 3] etc porphyrins exhibit red-shifted absorption bands and higher oscillator strengths than regular porphyrins, an effect caused by the fusion of the tetrapyrrolic macrocycle with external aromatic rings. Stronger red/near infra-red absorption allows for more efficient excitation of molecules in biological tissues at depth.
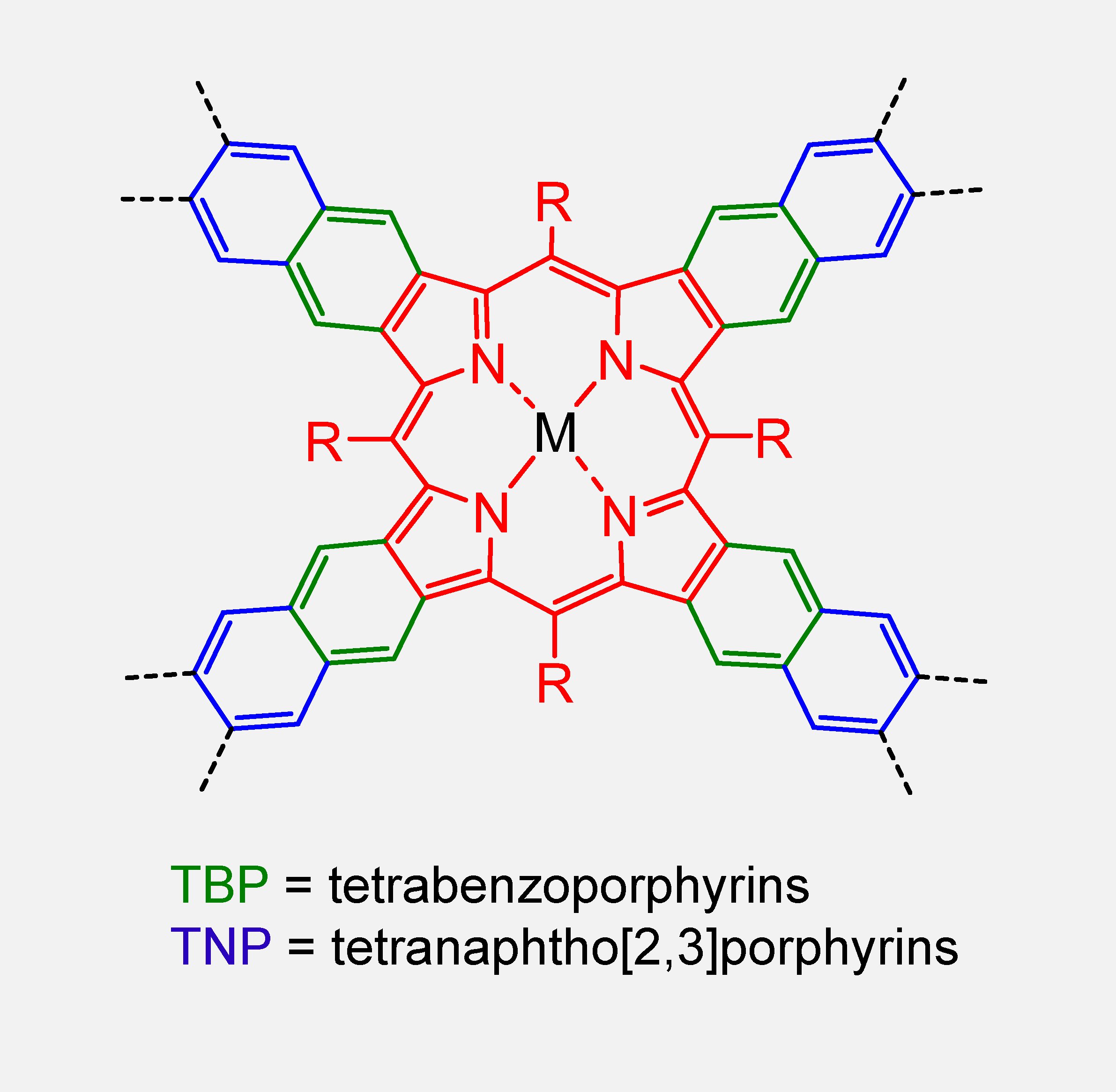 Laterally π-extended porphyrins.
Laterally π-extended porphyrins.
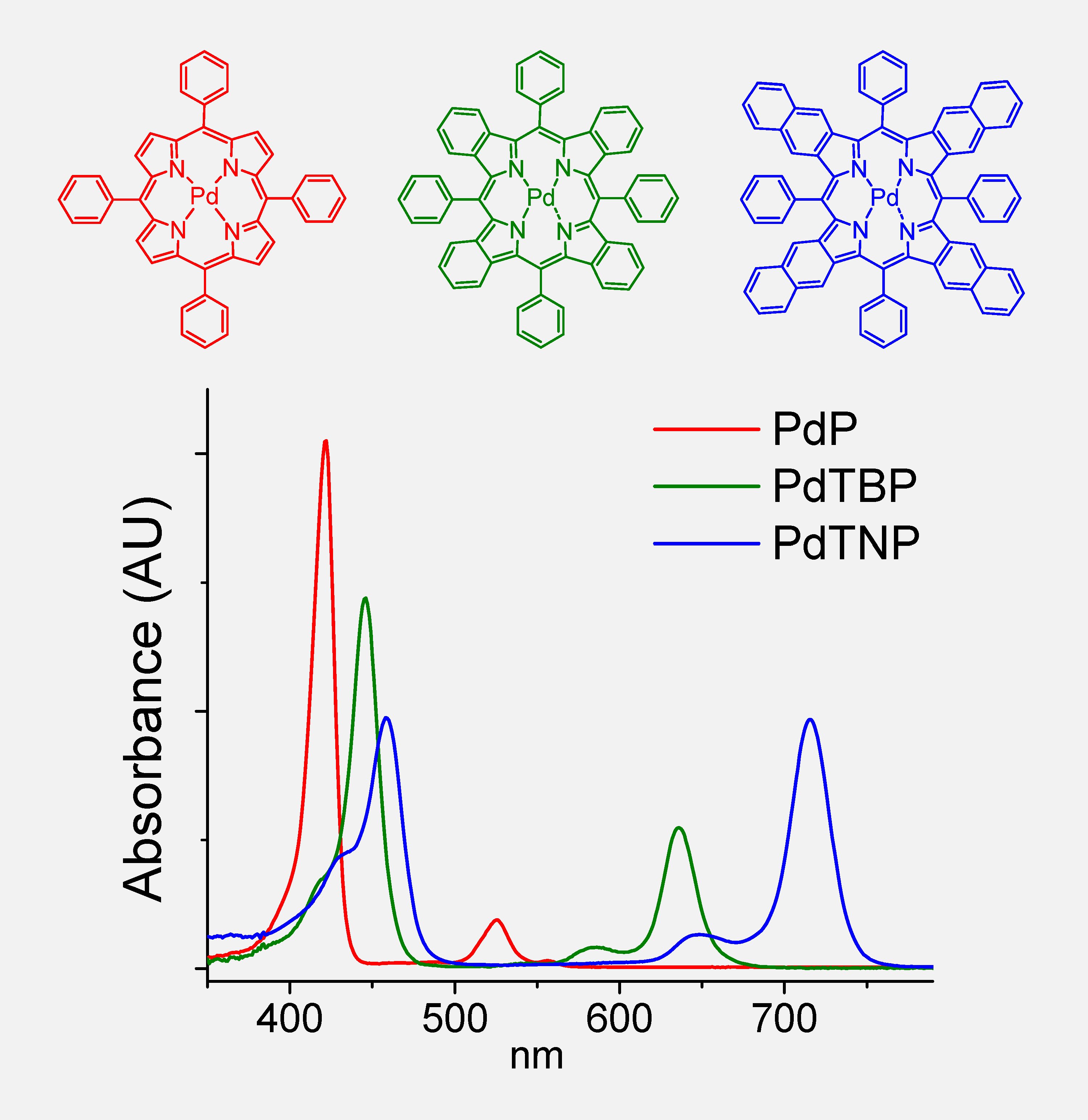 Changes in the absorption and phosphorescence spectra of Pd porphyrins upon aromatic π-extension of the macrocycle.
Changes in the absorption and phosphorescence spectra of Pd porphyrins upon aromatic π-extension of the macrocycle.
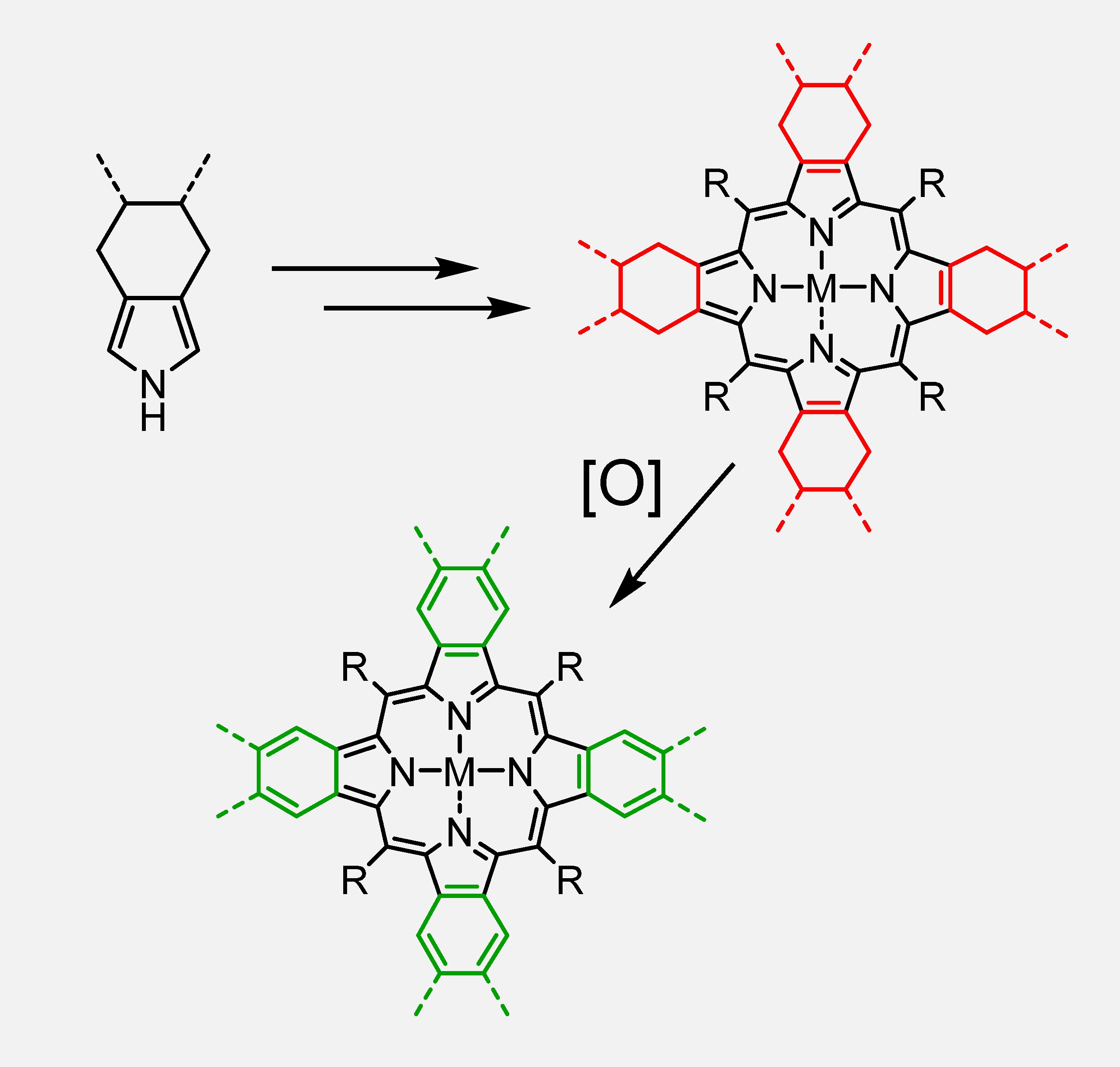 Synthetic approach to π-extended porphyrins via oxidative aromatization.
Synthetic approach to π-extended porphyrins via oxidative aromatization.
Methods of synthesis of porphyrins usually revolve around the condensation of pyrroles with aldehydes. Perhaps the most known example is the synthesis of porphyrins under equilibrium conditions developed by Jonathan Lindsey (NCSU). However, aromatically-extended analogs of pyrrole, e.g. isoindole or benzoisoindole, are thermodynamically unstable and cannot be easily used in porphyrin synthesis. Andrei Cheprakov proposed to assemble non-aromatically extended porphyrin-precursors first, using an array of previously developed methods, and then to carry out oxidative aromatization at the last stage of the synthesis. Over the years, many π-extended porphyrins have been synthesized using this general method with applications ranging from biosensing to organic light-emitting diodes (OLED).
2. Multiphoton absorption in porphyrins
Porphyrins have centrosymmetric (gerade or g-) ground
state wavefunctions. Consequently, the parity selection rules for absorption by
one-photon (1P) and two-photon (2P) mechanisms in closed-shell metalloporphyrins are mutually
exclusive. The lowest 1P-allowed states, B (Soret) and Q, are ungerade (u-) states, and cannot be accessed via 2P absorption (2PA). On the other hand, 2P-allowed
g-states in regular porphyrins lie at
such high energies that 2P excitation is impossible due to the interference of 1P transitions in the low-lying vibronic states of the Q band.
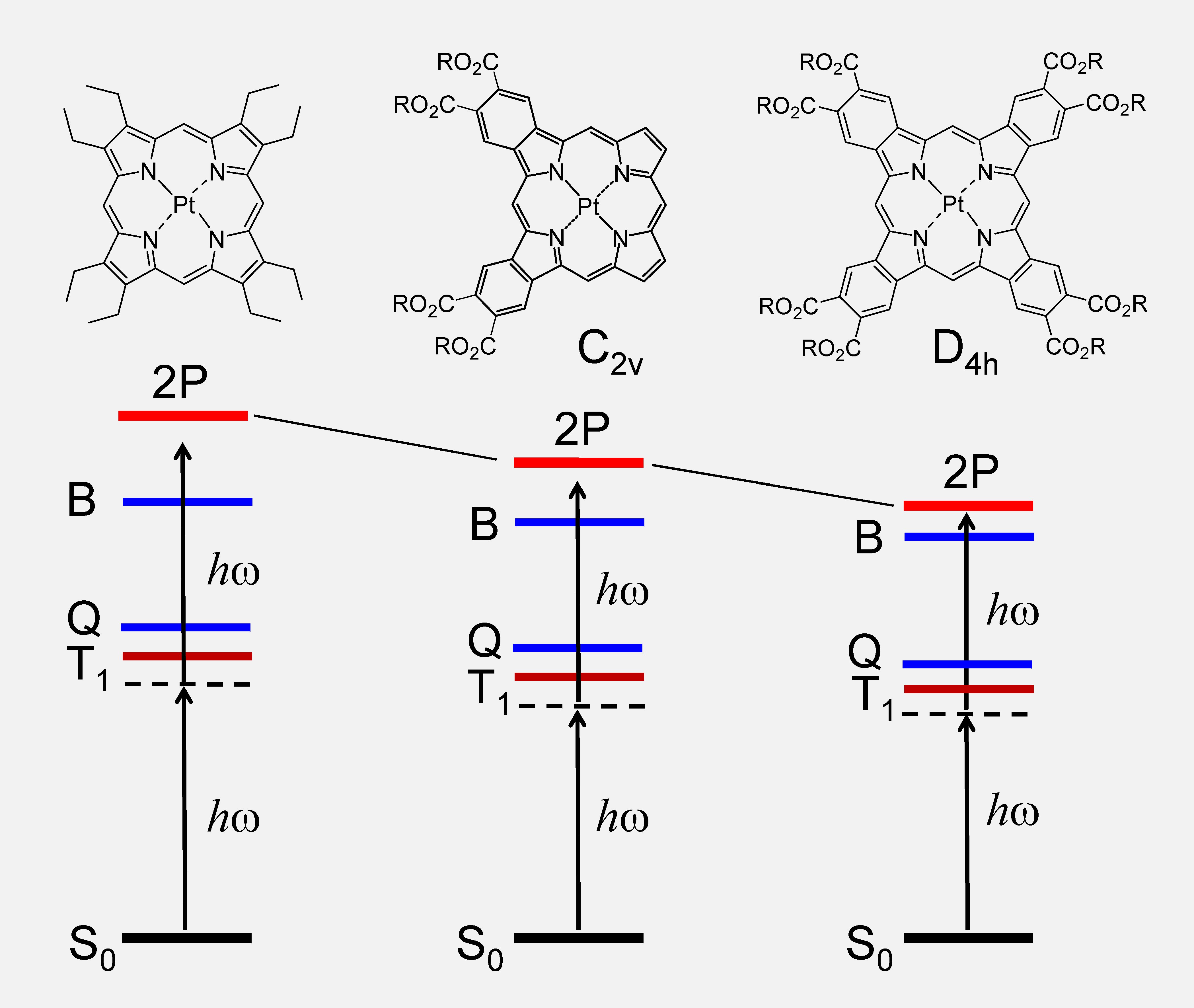 Effect of carbonyl groups on the energies of the 2P-allowed g-states in π-extended porhyrins.
Effect of carbonyl groups on the energies of the 2P-allowed g-states in π-extended porhyrins.
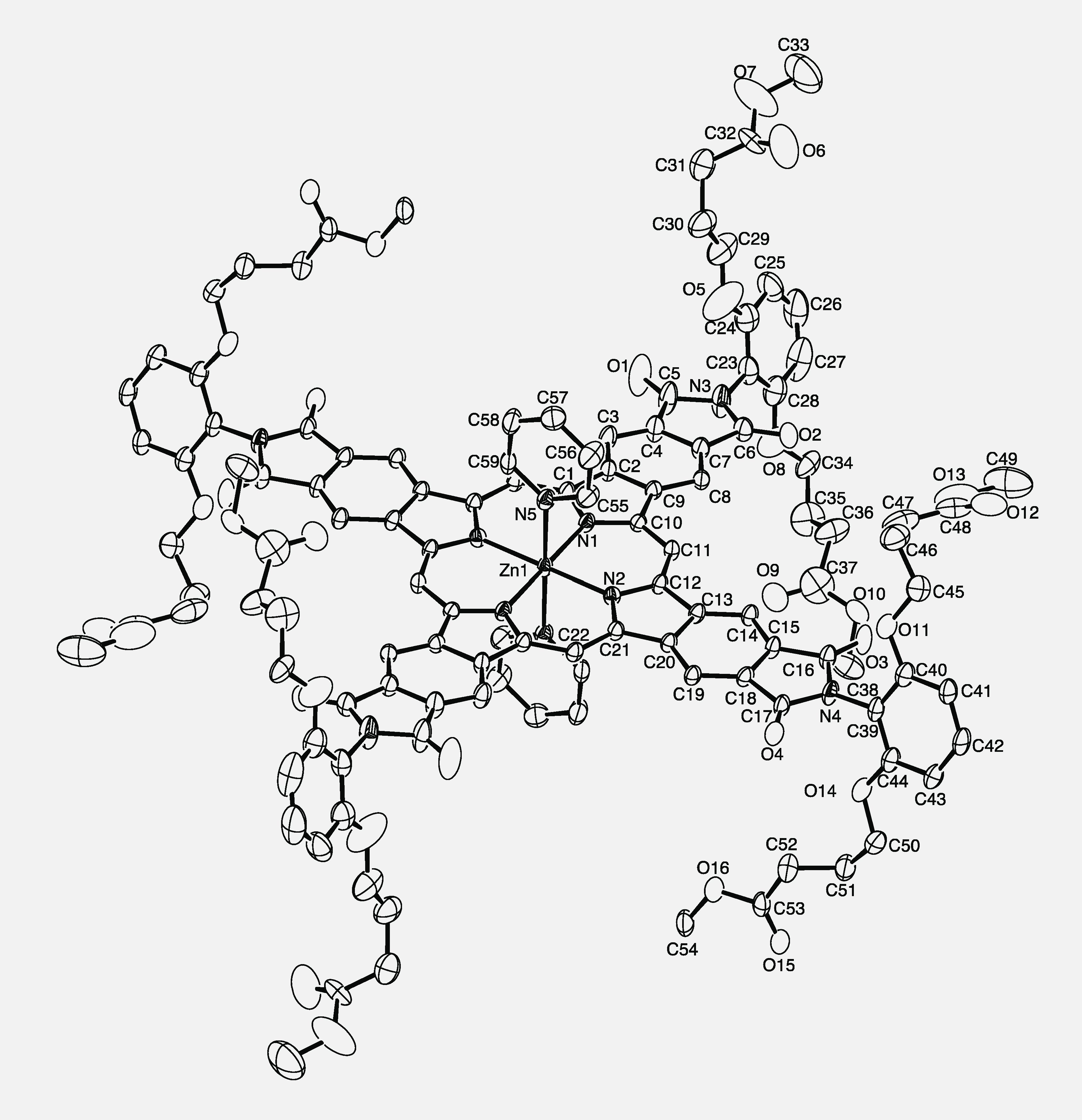 X-ray structure of Zn tetraarylphthalimido-porphyrin (ZnTAPIP).
X-ray structure of Zn tetraarylphthalimido-porphyrin (ZnTAPIP).
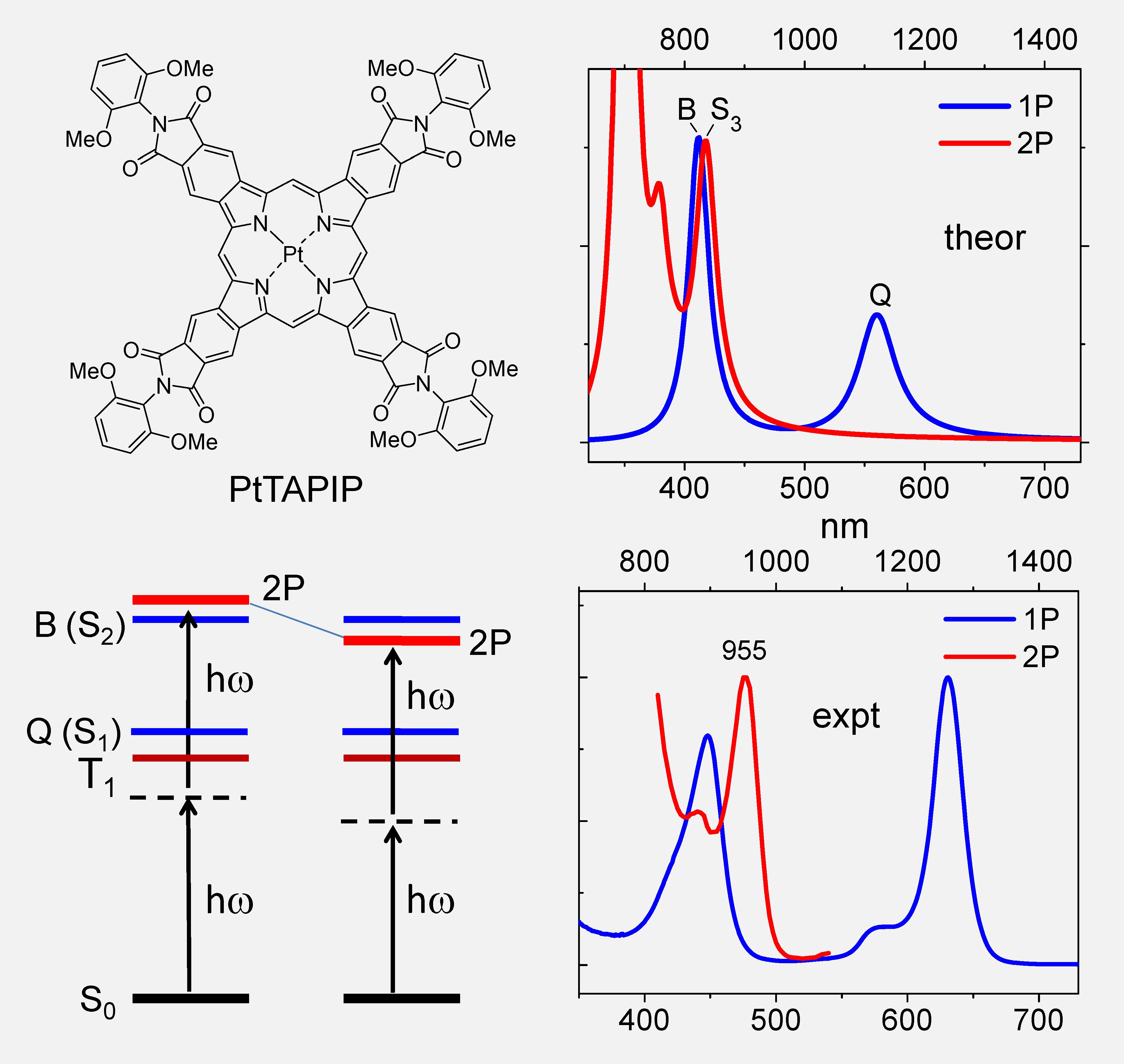 Computed and experimental 1PA and 2PA spectra of PtTAPIP.
Computed and experimental 1PA and 2PA spectra of PtTAPIP.
Recently we applied the oxidative aromatization method (see above) to synthesize asymmetric syn-dibenzo and syn-dinaphthoporphyrins [4] and found that the 'hidden' g-states can be stabilized by the substitution of the peripheral aromatic rings with electron-withdrawing groups [5]. This effect was found to exist in π-extended porphyrins irrespective of their symmetry, and in fact the most efficient 2P absorbers were found to be fully-symmetric tetrabenzoporphyrins possessing multiple carbonyl-containing substituents at the periphery. The Pt (II) complex of one such porphyrin, namely Pt tetraaryphthalimidoporhyrin (PtTAPIP) [6], was subsequently used to construct a high-performance two-photon oxygen probe Oxyphor 2P [7], which is presently used by a number of biomedical imaging laboratories.
Using experimental and computational methods we are presently studying the interplay between electronic structure and higher order absorption in porphyrins in an effort to create probes for three-photon and higher-order non-linear microscopy methods.
University of Pennsylvania
Department of Biochemistry and Biophysics, Perelman School of Medicine
Department of Chemistry, School of Arts and Sciences
Philadelphia, PA 19104
United States
The Vinogradov Group website
Copyright: VINOGRADOVLAB.ORG