Imaging probes
1. Phosphorescent probes for biological analytes
Phosphorescence is a spin-forbidden emission from triplet electronic
states of molecules (T1→S0), unlike the more common fluorescence (S1→S0), which
originates in singlet states. Triplet states typically have much
longer lifetimes than singlet states, i.e. microseconds vs nanoseconds, which presents a number of advantages for imaging.
The most prominent example of phosphorescent probes are probes for molecular oxygen (O2), which is a highly efficient quencher of triplet states. By measuring phosphorescence decay times of probes dissolved in biological fluids (such as blood plasma or interstitial fluid) concentrations of oxygen can be determined non-invasively and with high accuracy. This approach, known as the phosphorescence quenching method was originally developed by David F. Wilson [1, 2] at Penn, and is currently used in many areas of biomedical research.
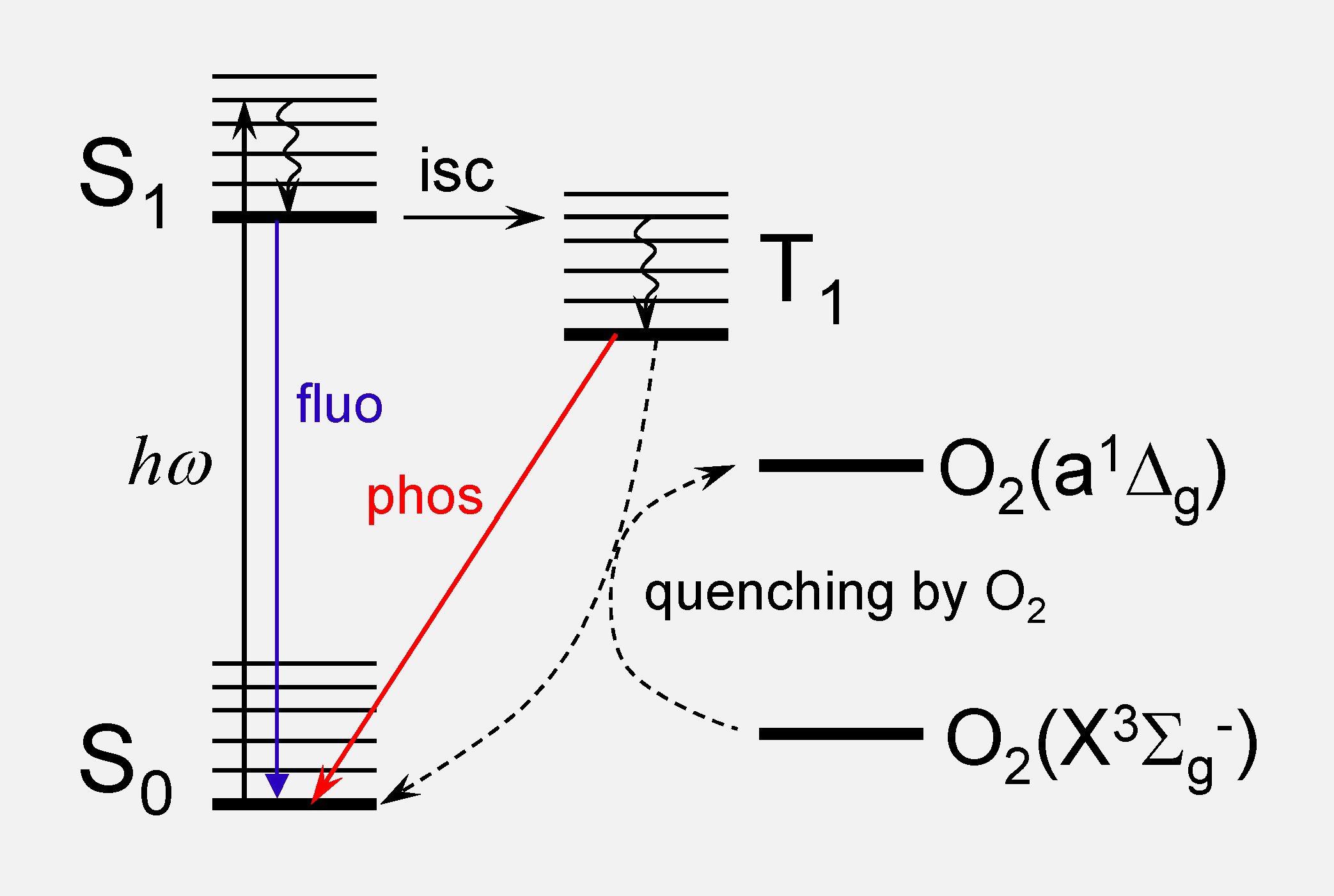 Energy diagram illustrating basic photophysical processes in a probe molecule and quenching of the triplet state by oxygen.
Energy diagram illustrating basic photophysical processes in a probe molecule and quenching of the triplet state by oxygen.
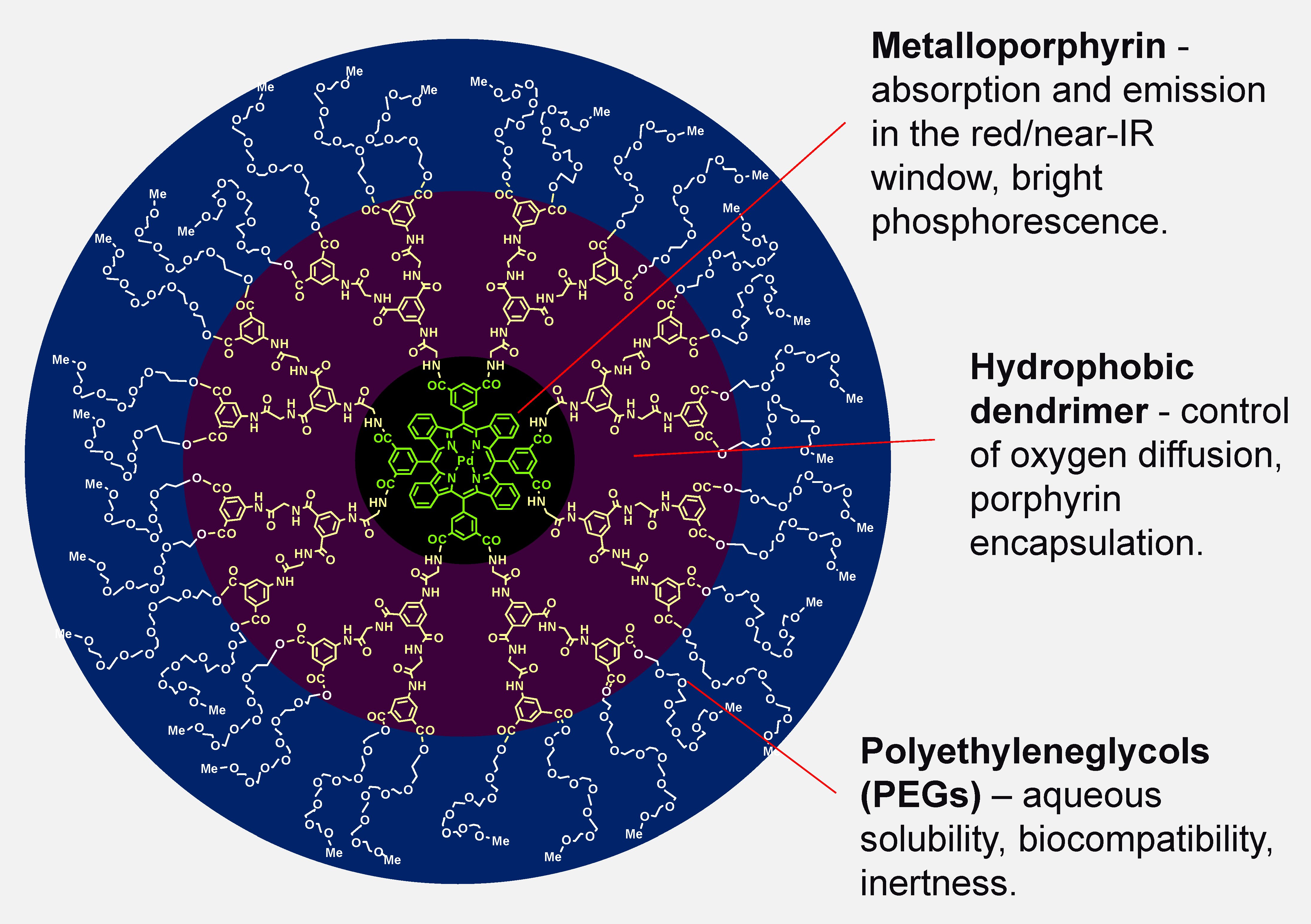 Structure of a dendritically protected phosphorescent probe for O2.
Structure of a dendritically protected phosphorescent probe for O2.
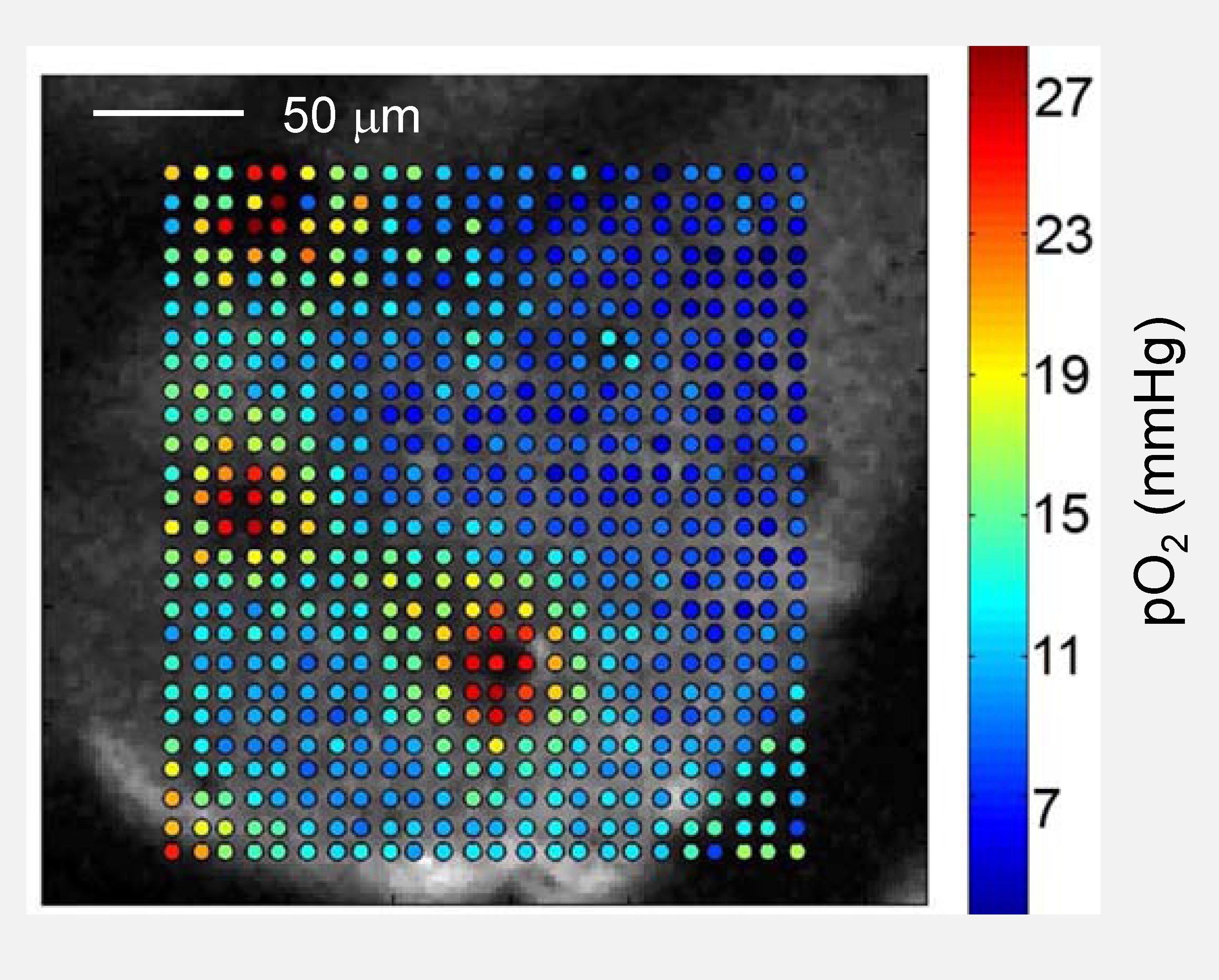 High-resolution oxygen map in the mouse brain obtained using 2PLM. Depth: 80 μm.
High-resolution oxygen map in the mouse brain obtained using 2PLM. Depth: 80 μm.
Our oxygen probes are based on metalloporphyrins, in which both the formation of triplet states via intersystem crossing (S1→T1) and the subsequent phosphorescence occur with high quantum efficiencies. To ensure biological compatibility and selectivity for oxygen, porphyrins are encapsulated within hydrophobic dendrimers, whose periphery is modified with polyethyleneglycol (PEG) residues [3, 4, 5 ]. The resulting constructs are inert, hydrophilic and fully bio-compatible. The phosphorescence lifetimes of these probes provide a selective signal for oxygen and allow measurements of absolute oxygen concentrations in any aqueous environment. Measurements based on phosphorescence lifetimes are independent of the probes' concentrations and unaffected by the optical properties of the medium.
Recently, the capabilities of the method have been extended by combining it with two-photon laser scanning microscopy. The resulting technique is known as two-photon phosphorescence lifetime microscopy (2PLM) of oxygen [6]. 2PLM makes it possible to assess tissue oxygen metabolism with high spatial resolution at depth in such areas as the brain cortex [7, 8], the retina of the eye [9,10], bone marrow [11, 12, 13] and newly growing bone [14], thus addressing biological questions for which non-invasive imaging of oxygen provides critical information not obtainable by any other method. More clinically relevant applications include measurements in the intestines [15, 16] and in tumors undergoing radiation therapy [17].
One major advantage of phosphorescence is its long lifetime, which allows for complete suppression of scattered excitation and tissue autofluorescence using microsecond time-gating. We are developing phosphorescent probes for quantitative imaging of temperature, pH and metal ions simultaneously with oxygen. These probes are constructed around multichromophoric systems, in which energy and electron transfer processes are tuned to render desired quantitative analyte-specific signals.
2. Up-converting nanoparticles (UCNPs)
Multiphoton microscopy is currently the tool of choice for deep-tissue imaging, particularly in neuroscience, where relevant physiological processes can be observed just a few hundred microns under the brain surface in rodents. However, two-photon excitation of conventional probes requires pulsed ultrafast lasers, which are expensive and may cause tissue damage due to the extremely high photon fluxes that they generate. At the same time, there is a class of agents, known as lanthanide upconverting nanoparticles (UCNPs), in which 2PA, or an even higher order absorption, can occur under low energy continuous wave (CW) irradiation. This presents major advantages for bio-imaging. We use dendrimerization to impart bio-compatibility of UCNP crystals and to couple their signals to the sensing of biological analytes [18]. Using dendritic UCNPs, we for the first time were able to perform depth-resolved high-resolution angiographic imaging in a live brain using two-photon CW excitation with photon fluxes 105-106 times lower than those used in conventional two-photon microscopy [19]. However, using UCNPs for quantitative analyte sensing in vivo still remains an unsolved problem and constitutes an active area of research.
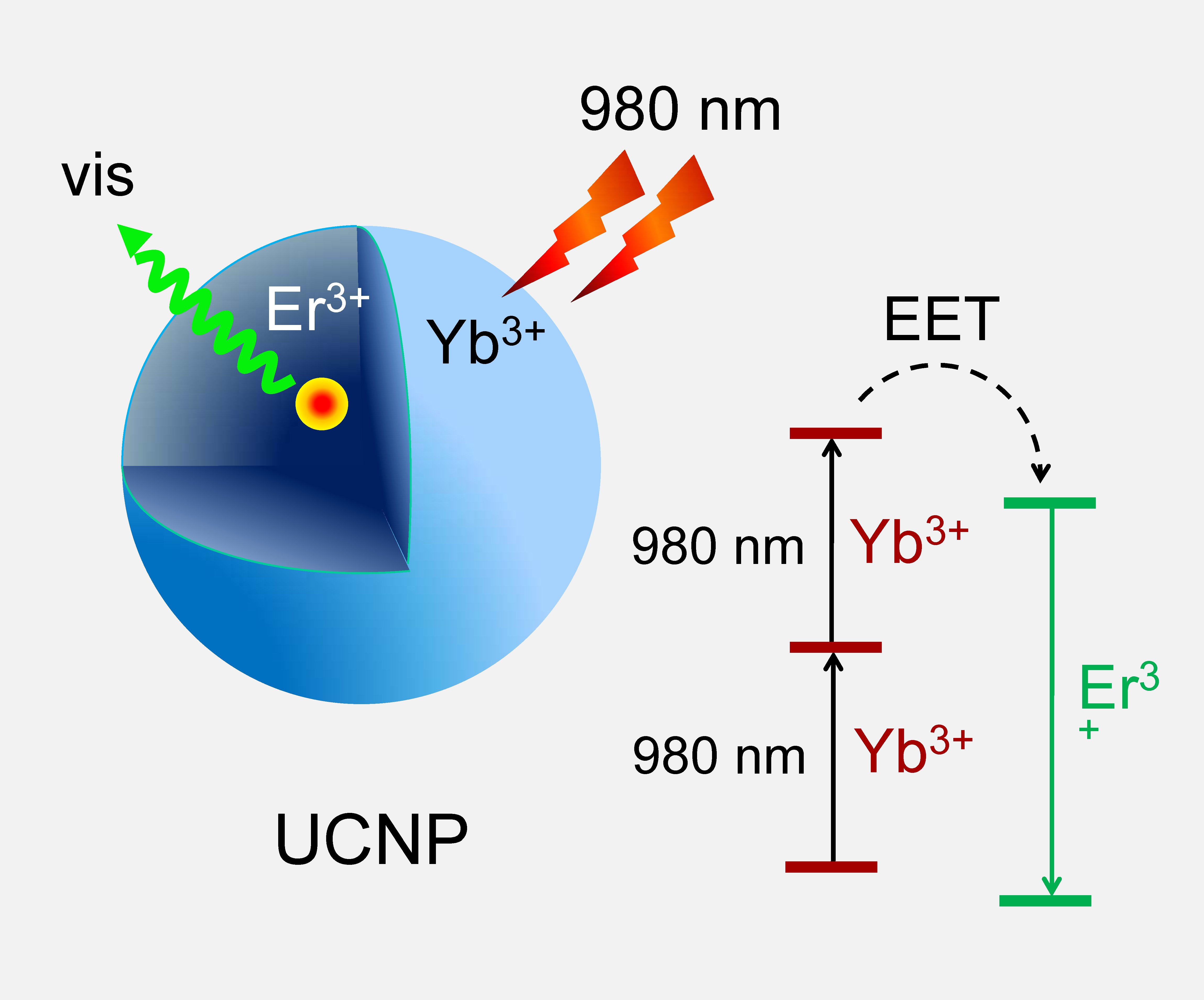 Sequential two-photon excitation and energy upconversion in UCNP crystals.
Sequential two-photon excitation and energy upconversion in UCNP crystals.
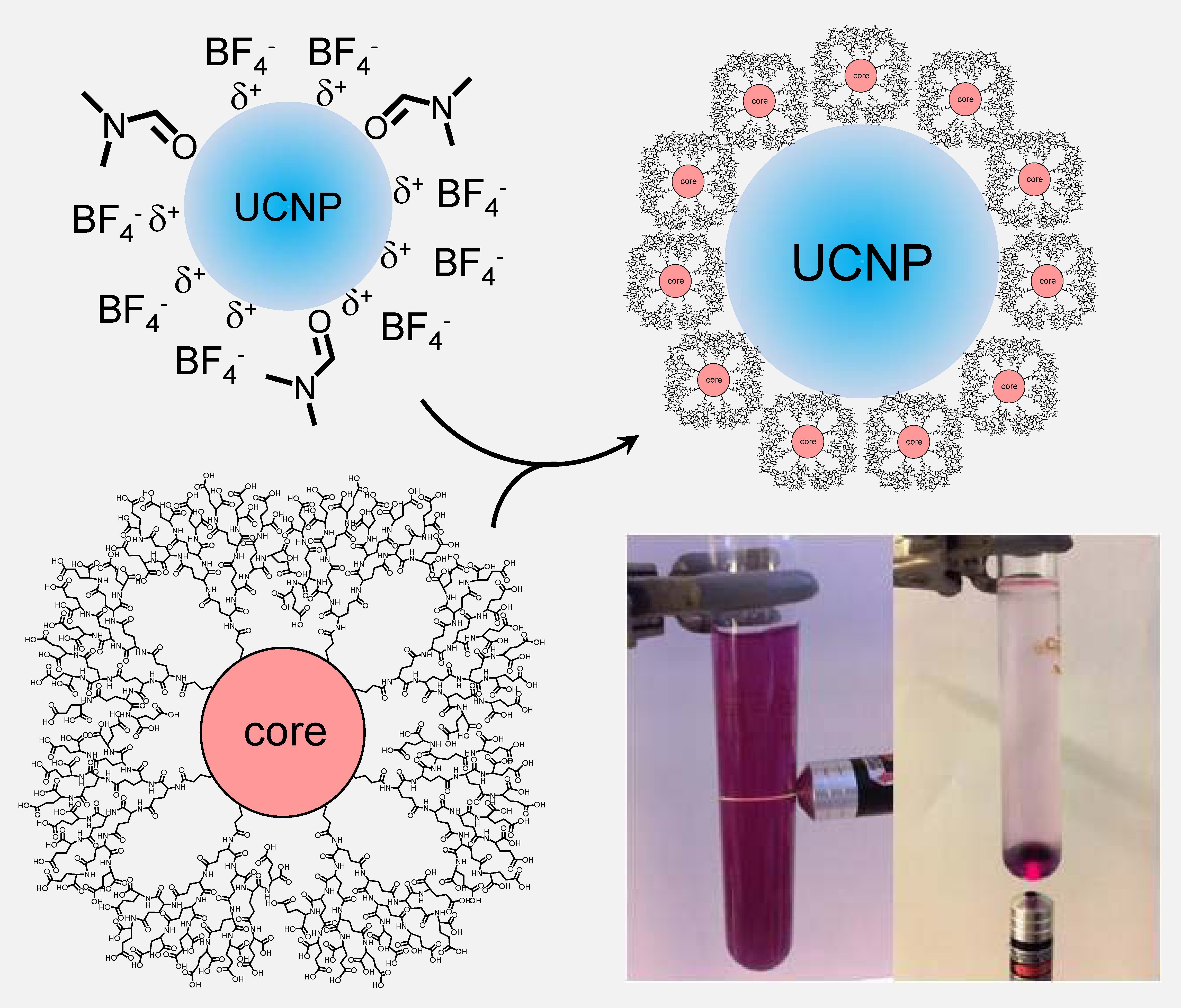 Solubilization of UCNPs using hydrophilic dendrimers. Upconverted emission from an aqueous solution of dendritic UCNPs excited by a hand-held laser pointer (980 nm).
Solubilization of UCNPs using hydrophilic dendrimers. Upconverted emission from an aqueous solution of dendritic UCNPs excited by a hand-held laser pointer (980 nm).
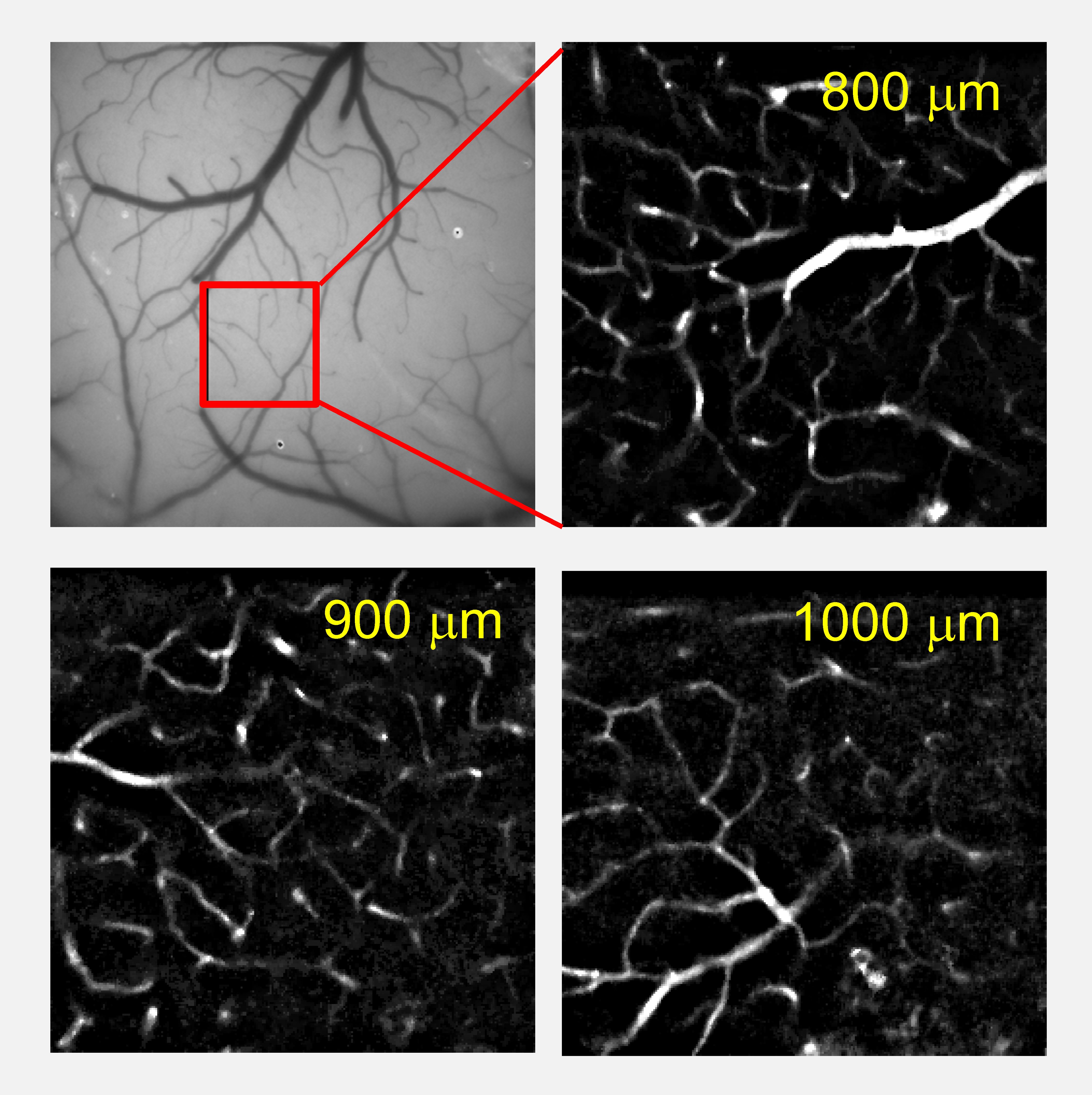 High-resolution depth-resolved maps of brain vasculature in mice obtained using dendritic UCNPs and two-photon CW excitation with average power not exceeding 20 mW.
High-resolution depth-resolved maps of brain vasculature in mice obtained using dendritic UCNPs and two-photon CW excitation with average power not exceeding 20 mW.
University of Pennsylvania
Department of Biochemistry and Biophysics, Perelman School of Medicine
Department of Chemistry, School of Arts and Sciences
Philadelphia, PA 19104
United States
The Vinogradov Group website
Copyright: VINOGRADOVLAB.ORG